mRNA-Based Therapies Beyond Vaccines
In today’s world, where the need for rapid and adaptable medical solutions is more critical than ever, mRNA stands at the forefront of scientific innovation, signaling a new era of medical breakthroughs and improved patient outcomes.
Usage of mRNA technology in developing COVID-19 vaccines has opened the door to a new era of medical innovation. mRNA technology can be used in a wide range of therapeutic fields, including targeted cancer treatments, genetic disorders, infectious diseases, and regenerative medicine, it has the potential to revolutionize healthcare. As research and development continue to advance, mRNA technology is poised to transform the landscape of healthcare, providing new avenues for personalized medicine and innovative treatments.
Personalized vaccinations that teach the immune system to recognize and target certain cancer cells are made possible by mRNA, offering a customized strategy that may improve therapeutic effectiveness while reducing adverse effects. By producing functional proteins, mRNA therapies could directly address the biological origins of diseases like muscular dystrophy and cystic fibrosis by replacing or repairing damaged genes in hereditary disorders.
By promoting the body’s natural healing processes, mRNA has the potential to treat illnesses including heart disease and spinal cord injuries by stimulating cells to create proteins necessary for tissue repair and regeneration. Fundamentally, mRNA acts as a blueprint that instructs cells to make proteins for therapeutic purposes, transforming contemporary medicine by facilitating more efficient, individualized approaches to disease prevention and therapy.
As research progresses, the potential of mRNA therapeutics continues to expand, offering new possibilities for precision medicine and individualized treatment approaches. With ongoing advancements in delivery systems, stability, and efficacy, mRNA technology is set to revolutionize the future of healthcare, providing next-generation solutions for some of the most challenging diseases.
The new technologies, particularly groundbreaking innovations like mRNA vaccines, should be patented to drive economic growth and foster a competitive edge in the global marketplace. Furthermore, analyzing mRNA-related patents can provide insights into technological advancements that supplement scientific literature, offering an overview of understandings industry trends and technological impact. By enabling companies to leverage exclusive rights to breakthrough technologies, patents stimulate economic growth, drive technological progress, and create incentives for continued innovation. Hence, this article offers an overview of the mRNA patent landscape and how it’s evolved in the past few years.
Patenting Trends in mRNA-based therapies
1. Multiple usages of mRNA
Figure 1 diagram provides an overview of mRNA-based therapies, such as vaccine compositions, triggering immune response, usage of lipid nanoparticles as carrier, cancer treatment, genetic disorders by replacing or repairing faulty proteins, regenerative medicine, manage autoimmune diseases and delivering genetic instructions to cells.
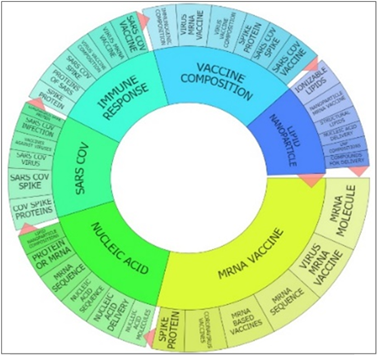
Figure 1: Various usages of mRNA technology
2. Global Patent Distribution
Figure 2.1 shows the percentage of distribution of patent activity in the top 10 jurisdictions. China and US countries has been ranked top in publication when compared to other countries. Whereas PCT (WO) and the United States (US) also have substantial portions, suggesting that PCT filings and US based patent activity is prominent. And, other regions, including Japan (JP), European Patent Office (EP), Australia (AU), South Korea (KR), Taiwan (TW), India (IN), and Russia (RU), contribute to mRNA technology.
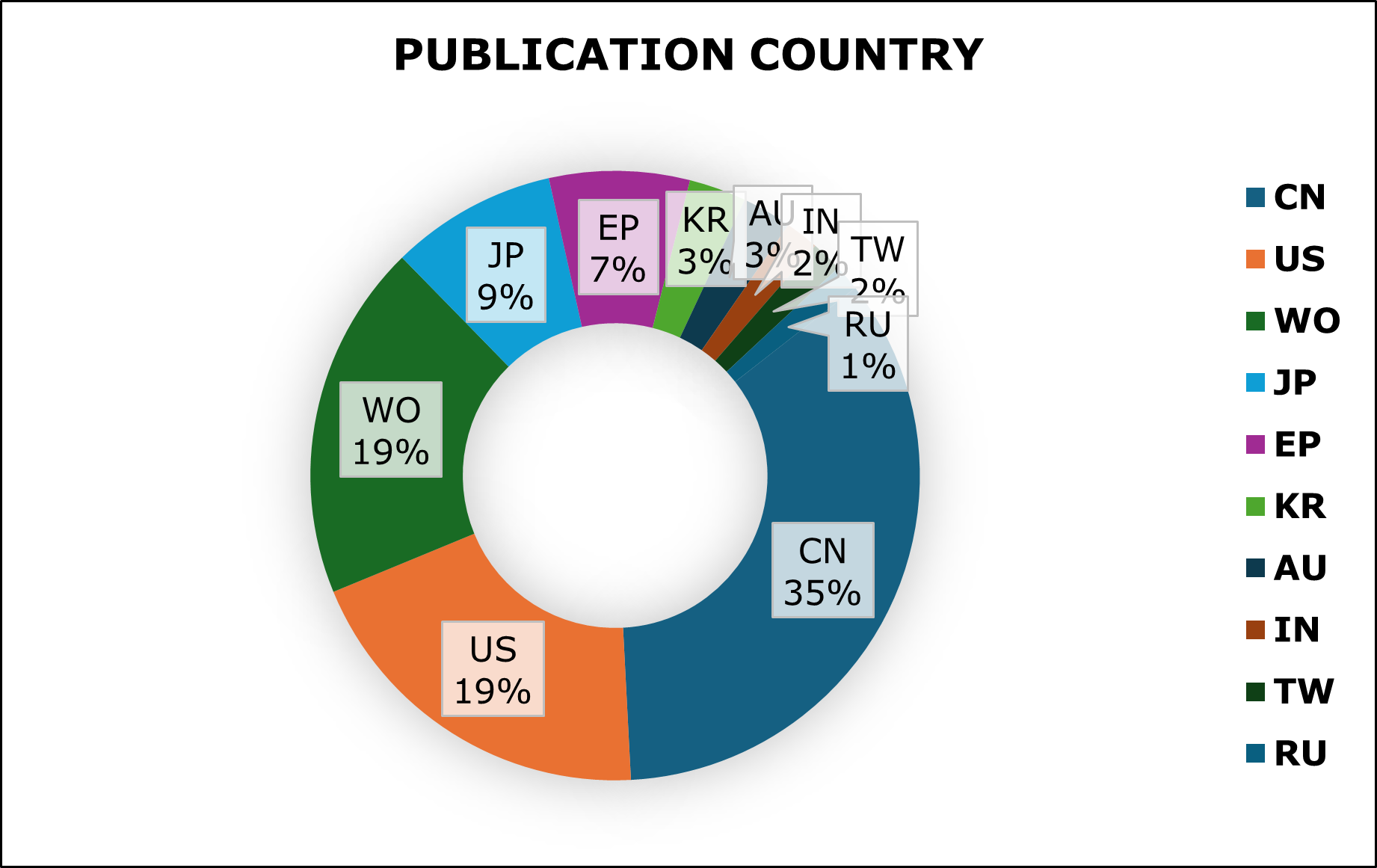
Figure 2: Geographical distribution of patent activity
3. Top Players in mRNA Technologies
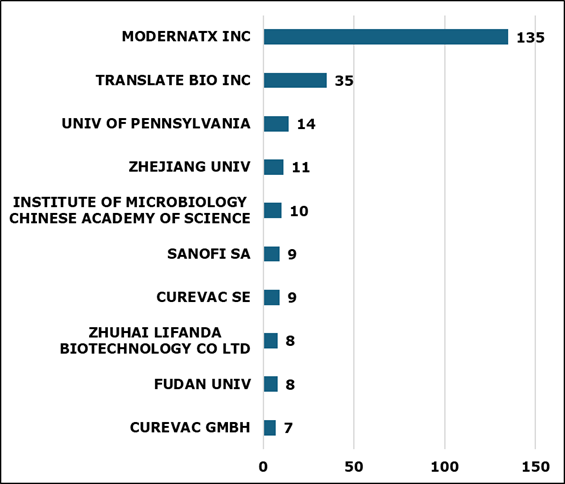
Figure 3 shows the top 10 leading contributors to mRNA technologies. Moderna is leading the technology with the highest number of patent filings followed by Translate Bio. Interestingly, the research institutions like University of Pennsylvania, Institute of microbiology, Zhejiang university and Fudan university are significantly outpacing the advancements in mRNA technology.
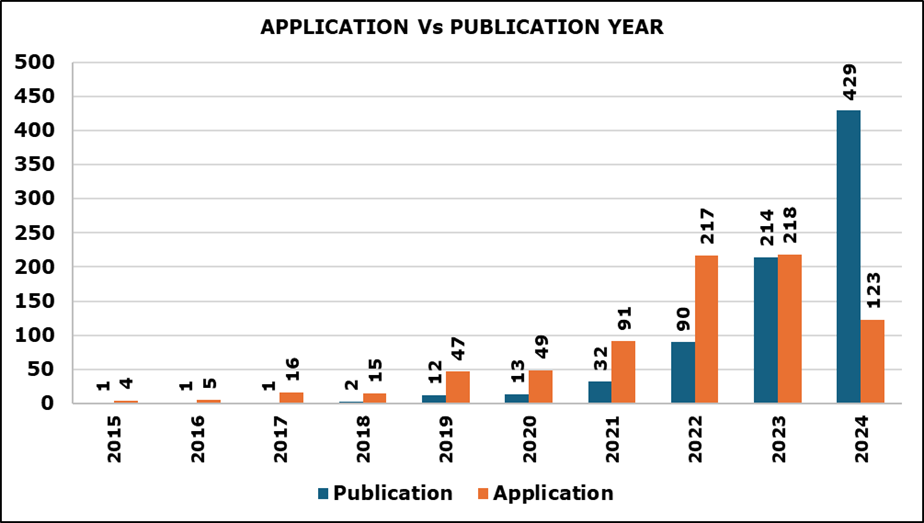
4. Evolution Over Time: Applications and Publications
Figure 4 shows the trend with the number of patents published per year from around 2014 to 2024. Between 2014 and 2018 approximately, the number of patents remains relatively low and steady. However, starting in 2019, there is a sharp increase in patent application and publication, with a drastic rise from 2020 onwards. The trend peaks in 2024, where the patent count exceeds, indicating a significant surge in patent activity over the last few years.
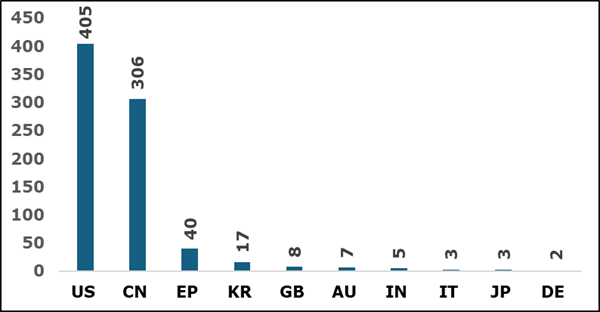
5. Priority Country for Patent Filings
Figure 5 illustrates showcasing the origin of mRNA-based patent filings across different jurisdictions. It reveals that the US and China dominate in mRNA patent filings.
Conclusion
The role of mRNA technology beyond its success in COVID-19 vaccines, has showed its transformative potential in various medical fields. It discusses applications in cancer treatment, genetic disorders, infectious diseases, and regenerative medicine, emphasizing how mRNA therapies can offer personalized and efficient treatment strategies.
It also includes statistical data and figures illustrating trends in mRNA-related patents, such as the rapid increase in filings since 2019, leading innovators (with Moderna Inc. ranking first), and the dominance of the U.S. and China in patent publications.
It is important to secure intellectual property right protection in the field of mRNA technology. The continuous rise in patent applications highlights the growing urgency and excitement surrounding this groundbreaking technology.
Disclaimer:
The information provided in this article is for informational and educational purposes only. It does not constitute legal, financial, or professional advice and should not be relied upon as such. While every effort has been made to ensure accuracy, the author and publisher make no representations or warranties regarding the completeness or reliability of the information presented. Readers are encouraged to consult with a qualified patent attorney or intellectual property professional for specific guidance related to patents and regulatory matters. Any opinions expressed are solely those of the author and do not necessarily reflect the views of any affiliated organizations or institutions.