Orange book and its Applications
What is the Orange Book?
Orange book is an electronically available free database having a list of drugs that the U.S. Food and Drug Administration (FDA) has approved as both safe and effective. It is easier for medical professionals, researchers to search for generic equivalents to brand name drugs, drug patents, and drug exclusivity. An orange book is a reliable tool for medical professionals and researchers for information about FDA approved drugs.
What are the main uses of Orange Book
- To retrieve information such as drug application date, approval date and list of related patents to find out whether the patents claims drug substance (DS) such as active pharmaceutical ingredient or polymorph and drug product (DP) such as method of treating, dosage, composition, and formulations, and also to find out the expiry dates of the corresponding patents.
- To search for the Innovators drug and approved corresponding generic drug information.
- It helps in taking important decisions/strategies for pharmaceutical companies whether to go into the market for sale or any modifications can be done to the existing drug and protects it by filing a patent.
- To identify patent exclusivity period and respective deadlines for any approved drugs (example: Orphan Drug Exclusivity-ODE – 7 years, New Chemical Entity Exclusivity (NCE) – 5 years, Generating Antibiotic Incentives -5 years, New Clinical Investigation Exclusivity- 3 years, Paediatric Exclusivity (PED) – 6 months, Patent Challenge (PC) – 180 days).
- To identify the polymorphs of innovators patented drug and challenge the innovator’s patented drug in order to get the benefit of 180 days market rights in the US by filing ANDA (PIV).
What information can be searched?
- To find approved drugs
- Search by Proprietary Name, Active Ingredient or Application Number
- Search by Applicant (company)
- Search by Dosage form (Ex: tablet or capsule)
- Search by the rout of administration (Ex: oral)
Example: Let us consider a drug Copaxone which is used to treat multiple sclerosis
Proprietary name/brand/trade name: Copaxoneor glatopa
Active- ingredient name: Glatiramer acetate
FDA application number: N020622.
The following information is retrieved by providing any of the above information
- To know if the approved drug is a prescribed drug Rx/OTC(Over-the-counter drug)/DISCN (discontinued drug)
- Dosage form (capsule/tablet/injectable/suspension)
- Route of administration of the drug (oral/injection/ Subcutaneous)
- Strength of the drug (mg)
- Therapeutic code (TE Code) generally denoted by two letter code example AB which means generic drug meeting the bioequivalence standards of an approved drug,
- RLD means Reference listing drug that means whether the new drug product is approved by FDA made findings of safety and effectiveness
- RS means Reference standard whether generic player submitted the application which must compare in vivo/in vitro bioequivalence depending on its dosage and where in applicant seeks approval of an ANDA application based on above studies.
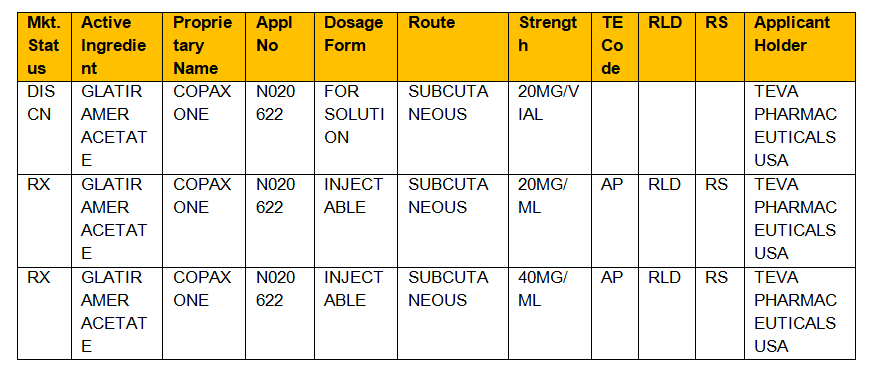
Orange book Table
- View newly added patents or delisted patents
- To find patent information
- Search by patent number
The information such as concerned drug application number, active ingredient, trade name, dosage form, route, strength, patent number, patent expiry date, drug substance (which claims substance/active ingredient) drug product (which claims formulation, composition, MOT/MOU), Patent Use Code to identify the use of a drug product, market status, and date of submission to FDA can be retrieved
Resources:
- Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations https://www.accessdata.fda.gov/scripts/cder/ob/
- Approved Drug Products with Therapeutic Equivalence Evaluations (Orange Book)
https://www.fda.gov/Drugs/InformationOnDrugs/ucm129662.htm
- One-Time Report on Marketing Status Required by FDARA
- Frequently Asked Questions on the Orange Book
https://www.fda.gov/Drugs/InformationOnDrugs/ucm114166.htm
- Resources for Information on Approved Drugs
https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/default.htm
- Orange Book Preface
https://www.fda.gov/Drugs/DevelopmentApprovalProcess/ucm079068.htm
- Novel Drug Approvals for 2019
https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugInnovation/ucm629491.html
